Researchers at the Janelia group created an innovative way for engineered protein biosensors & bright, fluorescent Janelia Fluor (JF) dyes to work together. Aim of the new biosensor is to measure physiological signals in live animals.
The revolutionary sensor, WHaloCaMP, can track multiple physiological signals in live animals, unlike its previous version. And when it comes to illumination, the detector can emit bright far-red light. This light can penetrate deeper into tissues than the other (existing) wavelengths.
This newly added waveband, supplements color beyond typical hues like green and red. Consequently, the effect can enhance the range of detectable signal during biological imaging.
Fluorescent Biosensors to Image Physiology
Things did not go well during the first shot. It failed when the sensors were tested in live animals, recalled Dr. Schreiter. Fortunately, Dr. Helen Farrants had just arrived at Janelia for her postdoc in Schreiter’s lab, and she decided to re-design the protein biosensors to carry out the original intention.
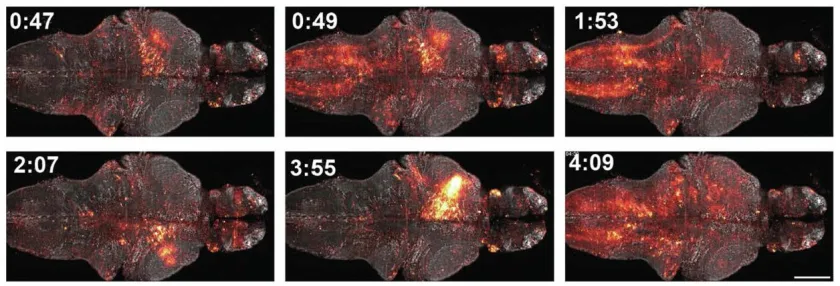
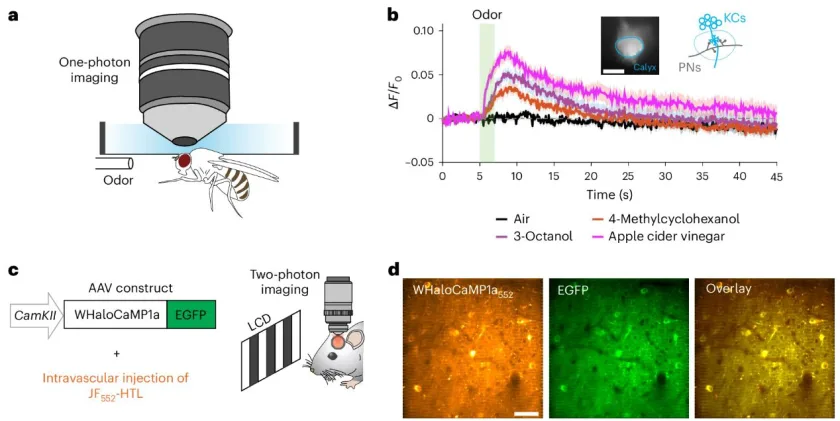
The re-designed strategy was to create a new method for protein biosensors to interact with JF dyes. The team created their first proof-of-principle sensor, which they termed as WHaloCaMP. This sensor was able to track calcium signals in live fruit flies, zebrafish, and mice.
The limitation of new sensor went far beyond detecting calcium signals. For instance, it also allowed for developing various sensors to track multiple physiological signals. This new biological insight would help in visualising functions of other cells, tissues and organs, along with their collaboration to perform mutual functions.
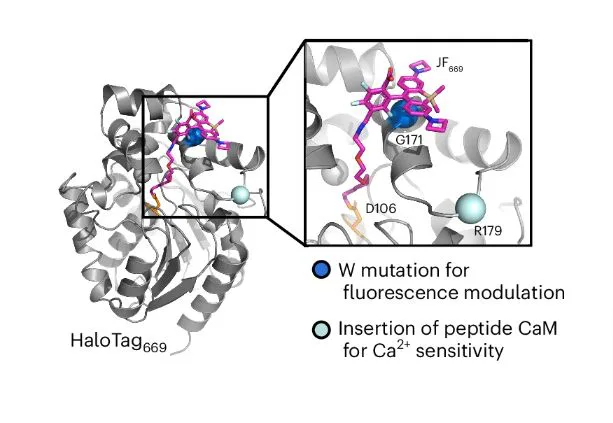
So, how did Farrants and the team figured out the way to club the protein biosensor and the JF dye?
During its initial phase, the dyes relied on form changes to fluorescent, which means, dye had to alter its physical configuration to emit light. Once clubbed with sensors, these dyes failed to enter animal tissue, hence no signal was traced in live animals.
After exploring strategies for over a year, Dr. Farrants and team came up with an innovative approach. They targeted particular parts of the sensor protein to control fluorescence. Hence, they totally replaced their dependence on dye for form change. And then added tryptophan, an amino acid, near the dye on the sensor protein. This acted as a control switch to on and off the fluorescence.
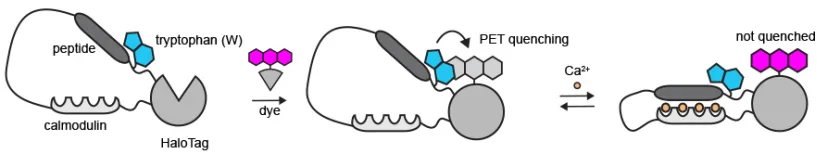
How? – The Tryptophan Change
In absence of calcium, the tryptophan stays close to dye and the dye turns off. While in presence of calcium, the protein changes shape and tryptophan move away, consequently, the dye turns on.
After struggling for a year, the results showed no improvement but when the team made tryptophan change, they observed slight change in fluorescence after adding calcium. That was the turning point for the entire research, recalled Dr. Farrants.
WHaloCaMP can Detect & Visualize Calcium Signals
The researchers showed that WHaloCaMP can detect calcium signals in live fruit flies, zebrafish, and mice. Not only this, the modular chemigenetic calcium indicator, can also trace at least three signals simultaneously with the help of different colors. For instance, in case of zebrafish, WHaloCaMP detected:
- glucose changes
- muscle, and
- neuron calcium signals.
The research does not stop here, they are is working towards fabricating more sensors for other physiological signals and creating additional sensors using JF dyes.
Takeaway
The future of WHaloCaMP looks promising as it could lead to more high-end and versatile tools for biological research. Tracing molecular and cellular activities would surely open doors for more in-dept analysis and better understanding of fundamental mechanisms in health and disease.
It’ll also imply that in near future we will have personalized medication tailored specifically for patient-specific requirements.
Source: Janelia Research Group