Generally, bacteria are envisioned as tiny, single-cell organisms scattered thinly on surfaces or floating in liquids. However, in various settings, bacteria prefer to grow in clusters known as biofilms. The formation of biofilms provides certain advantages to bacteria. In these clusters, bacteria can collaborate and create a protective environment that enhances their survival and growth.
Biofilms, while aiding in kombucha tea production, present a challenge by complicating the control of bacterial growth. When bacteria form a biofilm, it acts like a protective shield, making the cells resistant to antibiotics.
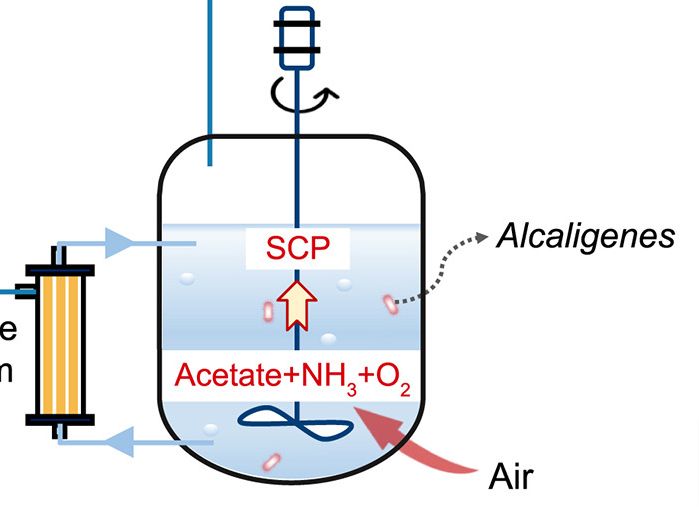
Kombucha tea is a fermented beverage made through the fermentation of sweetened tea with the help of a symbiotic culture of bacteria and yeast (SCOBY). This process results in a fizzy, slightly tangy drink that may offer various potential health benefits due to the presence of probiotics and organic acids.
Biofilms have Organized Structures
Until now, scientists thought bacteria in biofilms were kind of randomly scattered. However, recent research from Professor Lars Dietrich at Columbia University shows that bacteria in biofilms actually have a super organized structure within those gooey matrices.
Bacteria forming biofilms face a bit of a yin-yang situation, explained Professor Lars Dietrich. On one hand, the biofilm shields them from antibiotics and other dangers. But on the flip side, it keeps food out, making it tough to sustain the system.
So, as per Prof. Dietrich this research lays the groundwork for understanding how to shape the arrangement of bacterial cells. It also guides us in finding ways to make them more receptive to antibiotics.
Vulnerability to Surface Mutations
The team took common pathogen, called Pseudomonas aeruginosa into consideration. And utilized scanning electron microscopy and fluorescence microscopy, combined with cell labeling, for their study.
They found that within biofilms, P. aeruginosa cells are tightly packed lengthwise and oriented perpendicular to their growth substrate. This substrate serves as both their living space and the source of sustenance for their survival and growth.
Additionally, they observed that mutations affecting the bacterial cell surface can disturb this organized arrangement.
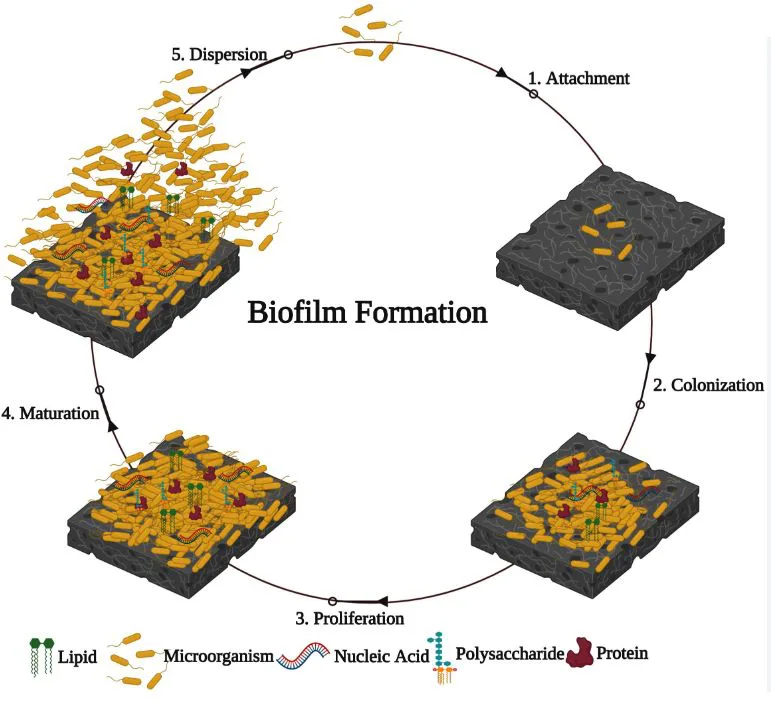
Biofilms Impact Sensitivity to Antibiotics
During their experiments, when the team introduced an external sugar to a fully developed biofilm, they noticed that its spread was influenced by the structure of the biofilm.
Bacteria with altered cellular arrangements, due to mutations, showed heightened sensitivity to added sugar or antibiotics in specific sections of the biofilm, known as subzones.
In the end, they proved that changes in the structure of the biofilm affect where the highest metabolic activity occurs within the biofilm.
Shaping Strategies for Enhanced Pathogenic Infection Control
In summary, these findings suggest that the microstructure of biofilms can be adjusted to impact the metabolism of specific bacterial subpopulations. This eventually influence the overall survival of the entire group.
This also has important implications for how we approach the treatment of infections caused by biofilm-forming pathogens like P. aeruginosa. Understanding and manipulating biofilm microstructure could open new avenues for developing more effective strategies against these infections.
Takeaway
Scientists have been looking for ways to gain insights into biofilm microstructure. As its future implications are significant and extend across various fields. Some of them are as follows:
- By adjusting the microstructure, researchers may enhance the susceptibility of biofilm-encased bacteria to antibiotics. Thus, it can improve treatment outcomes.
- By manipulating biofilm microstructure, researchers can design infection control strategies in areas where biofilm-forming pathogens pose persistent challenges.
- Future applications may involve designing medical devices with surfaces that resist or disrupt biofilm formation. Thus, reducing the risk of device-associated infections.
- Understanding and manipulating biofilm microstructure could also lead to improved environmental engineering practices for water treatment and pollution control.
In summary, the future implications involve not only improving our understanding of biofilms but also leveraging this knowledge to revolutionize approaches in medicine, environmental science, and biotechnology. The potential applications span from targeted therapies to innovative engineering solutions, with the overarching goal of addressing challenges posed by biofilm-related issues in various domains.
Via: Columbia University