Zinc batteries have been explored as an alternative to lithium-ion batteries for large-scale energy storage since long.
Zinc-based batteries is preferred over li-ion batteries because it is abundant, low-cost, and environmentally friendly compared to other metals. However, their efficiency has been limited due to issues with the zinc metal anode.
However, with the recent development of a new electrolyte that improves the efficiency of the zinc metal anode to nearly 100%, researchers envision that it could make zinc batteries a viable alternative.
Zinc metal batteries over lithium-ion batteries
As the world continues to transition to renewable energy sources, there is an increasing need for efficient and reliable energy storage technologies.
Solar and wind power are intermittent energy sources, meaning that their output varies depending on weather conditions. This makes it essential to have energy storage systems in place to ensure a consistent supply of electricity.
According to Xiulei “David” Ji, Professor of Chemistry at Oregon State University, Zinc metal batteries have the potential to be a cost-effective and environmentally friendly than lithium-ion batteries. And with the development of the new electrolyte, their improved efficiency is a significant step forward in making them more accessible to consumers.
What is a battery?
The basic components of a battery are:
- An anode – an electrode through which electrons flow out of the battery and into an external circuit.
- A cathode – the electrode that acquires electrons from the external circuit and completes the flow of current.
- An electrolyte – the medium that separates the electrodes and allows the flow of ions between them, which is necessary for the battery to function.
In a battery, chemical reactions occur at the anode and cathode, which convert chemical energy into electrical energy.
During discharge, the anode undergoes oxidation, losing electrons and becoming positively charged. The electrons flow out of the anode and through an external circuit to the cathode, where they are used to reduce the cathode material, producing electrical energy.
During charging, the process is reversed. An external electrical source is used to drive electrons back into the anode, which reduces it and stores energy in the battery.
The electrolyte plays a critical role in this process by allowing ions to move between the anode and cathode, enabling the chemical reactions that produce and store electrical energy.
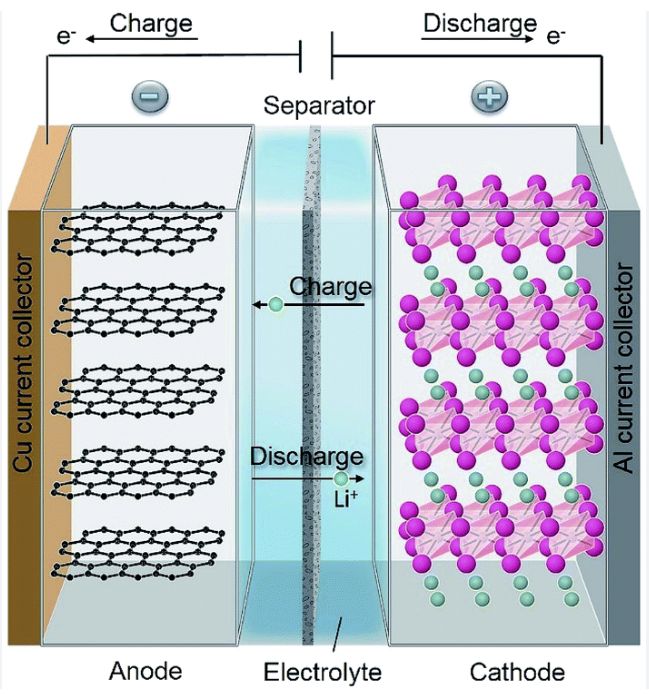
Zinc batteries for sustainable development
One of the advantages of zinc-based batteries is that they rely on a metal that is safe and abundant, unlike the metals used in lithium-ion batteries such as cobalt and nickel, which are becoming increasingly scarce and expensive.
Zinc is one of the most abundant elements on Earth, and its production is not associated with the environmental and social problems that are often associated with the production of rare metals.
Furthermore, the use of toxic metals like cobalt and nickel in lithium-ion batteries poses environmental and health risks. These metals can be harmful if they leach out of batteries and contaminate ecosystems and water sources.
The widespread adoption of lithium-ion batteries for energy storage could exacerbate these issues, making zinc-based batteries a more sustainable and responsible alternative.
The new hybrid electrolyte
The use of water and an ordinary battery solvent in the electrolyte is a key innovation, added Dr. Ji. The solvent makes the electrolyte non-flammable and of low environmental impact unlike the current, lithium-ion batteries.
The use of a mixture of inexpensive chloride salts, with the primary one being zinc chloride, also makes the electrolyte more affordable and accessible.
This is an important consideration for the commercial viability of zinc-based batteries, as cost is a key factor in determining their competitiveness with other energy storage technologies.
Highly efficient and cost-effective solution
The new electrolyte developed by Ji and collaborators has been shown to significantly improve the efficiency of zinc-based batteries.
The researchers were able to achieve a Coulombic efficiency (CE) of 99.95%, which is a measure of how effectively a battery can convert stored energy into electrical energy without losing energy to unwanted side reactions. Thus, making it a highly efficient and cost-effective solution for large-scale energy storage.
Takeaway
Efficient and cost-effective energy storage is a critical component of the transition to renewable energy sources. And zinc-based batteries offer a promising alternative to lithium-ion batteries, which have some limitations in terms of safety, cost, and availability of raw materials.
The breakthrough is an important milestone that could have significant implications for the future of energy storage and the transition to a more sustainable energy system.