In a breakthrough discovery, a new thing in cell biology called a hemifusome has come to light. It showed up when researchers used cryo-electron tomography (cryo-ET), it’s one of the ways to take super-detailed 3D images of cells while they’re still in a fairly natural state. Unlike traditional electron microscopy, which involves a lot of processing that can mess with delicate structures, cryo-ET keeps things closer to how they actually are in living cells. It was Dr. Seham Ebrahim and her team who used this method to uncover hemifusomes in four different mammalian cell lines, opening up a whole new view of how membranes fuse and vesicles form inside cells.
Using this method, researchers looked at four different mammalian cell lines:
- COS-7: A monkey kidney cell line often used to study cell signaling and protein expression.
- HeLa: A human cervical cancer cell line that’s widely used in biomedical research.
- RAT-1: A rat fibroblast cell line commonly used to study cell growth and transformation.
- 3T3: A mouse fibroblast cell line often used to explore cell cycle and differentiation.
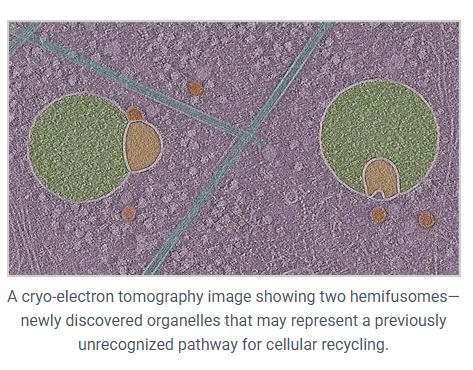
What they found was something no one had described before, which is, these odd vesicle pairs stuck together in a very specific way. They named them hemifusomes.
What actually is a hemifusome?
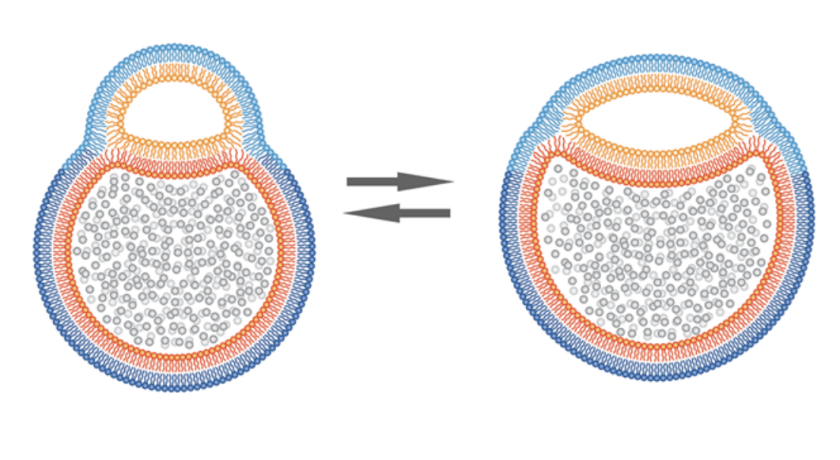
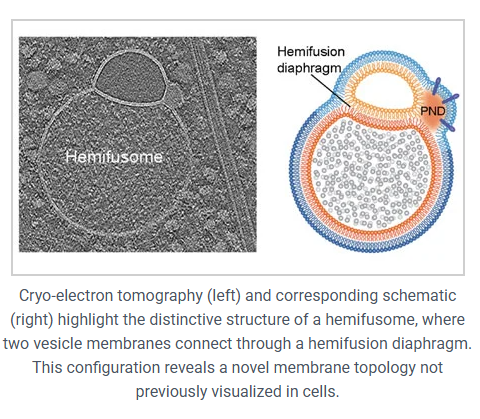
In simple words, it’s two vesicles that are partially fused together, but not all the way. They’re joined by something called a hemifusion diaphragm, which is just a flat patch of shared membrane between them. Up until now, people thought these kinds of structures were super tiny, about 10 nanometers, and would only last for a moment during full fusion events.
However, these are nothing like that. The hemifusion region in hemifusomes is about 160 nanometers across, and it sticks around. So we’re looking at a stable, long-lived structure that’s just been missed until now, probably because standard imaging techniques distorted or destroyed it.
Two kinds of hemifusomes show up:
- A small vesicle attached to the outside of a larger one
- A small vesicle embedded inside the larger one
In both cases, the smaller vesicle has a strange look to it, it’s translucent, almost completely smooth inside with no granules, or no clumps. This is unusual as most vesicles in cells carry proteins, enzymes, or other visible cargo. The only time they’ve seen a similar kind of translucent content before was inside multivesicular bodies (MVBs), but even then it was rare.
There’s also something else going on at the hemifusion sites: tiny droplets
Each hemifusome has what the researchers call a proteolipid nanodroplet, or PND. These are little spheres, about 42 nanometers wide, embedded right in the membrane where the vesicles meet. They aren’t just sitting there, they’re tucked inside the lipid bilayer itself.
They contain both protein and lipid components and pop up consistently across all hemifusomes. They also show up on their own, floating in the cytoplasm or embedded in other membranes. That kind of consistency usually means they’re doing something important.
The researchers think these PNDs might be starting points for hemifusome formation. Like little kits of material that help build a vesicle and attach it to another one.
So, what are these things doing?
That’s still a bit of an open question but here’s the idea the authors propose:
Rather than forming through the usual pathways we’ve known, like endocytosis involving clathrin or the ESCRT machinery, hemifusomes could represent a separate system for building new vesicles. Specifically, they might be involved in forming MVBs, but in a way that doesn’t require the ESCRT complex, which is normally considered essential for that process.
Here’s how it might work:
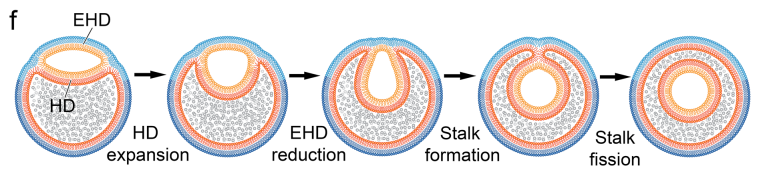
- A PND forms or floats near an existing vesicle
- It attaches and begins forming a new translucent vesicle within or beside the existing one
- This new vesicle hemifuses to the original, creating a hemifusome
- Eventually, that internal vesicle might fully pinch off, forming an intraluminal vesicle
They also found cases where multiple vesicles were hemifused together, like compound hemifusomes. These might act as hubs where several new vesicles are generated at once.
Are these involved in normal endocytosis?
Apparently not. They tested this by introducing gold nanoparticles coated with transferrin, which normally get pulled into the cell through standard endocytic pathways. The hemifusomes didn’t take any of them up. That suggests these aren’t just weird versions of endosomes, they’re doing something else entirely.
Also, they didn’t find any free-floating translucent vesicles in the cytoplasm, which supports the idea that these aren’t fusing and then floating off, they’re staying attached.
How common are they?
More common than you’d expect for something completely unknown until now.
In the thin areas of the cell periphery (which is where cryo-ET works best), hemifusomes made up about 10% of all observed vesicles. And they showed up across all four cell types.
So it’s not just some weird glitch, they’re actually a normal part of cell structure, just one that people missed ‘cause they’re hard to image with traditional methods.
Why it matters
This discovery expands how we understand membrane behavior inside cells. Up until now, long-lived hemifusion states weren’t really considered part of how cells organize or traffic vesicles. That might need to change.
If hemifusomes are involved in vesicle formation, cargo sorting, or lipid recycling, it could have implications for how cells handle stress, signaling, or degradation. A lot of diseases—including neurodegenerative ones—are tied to problems with membrane trafficking, so knowing more about alternate pathways is important.
Limitations and Open Questions
Most of the data came from thin parts of the cell near the edge. We don’t yet know if hemifusomes are common deeper inside or how their behavior changes in different conditions. There’s also a chance they show up more under stress (since the cells are slightly disturbed during sample prep), but the researchers think they’ve always been there and just weren’t visible with standard methods.
Takeaway
Hemifusomes are likely a regular part of the cellular membrane system. They’ve been hiding in plain sight, missed because they don’t show up well with conventional imaging. Now that cryo-ET can catch them, we’re starting to see that cells might have an entirely different way of handling membrane fusion and vesicle formation than we previously thought.
There’s still a lot we don’t know. But this opens the door to new questions and new directions in cell biology, like:
Could there be other long-lived hemifused states quietly doing work in parts of the cell we haven’t looked closely at? And how many other cellular structures are we still missing, just because our tools haven’t been good enough to see them yet?
Source: University of Virginia