At the fundamental level, function of human cell is to convert nutrients into energy and useful products that sustain life.
In the process, these cells give out certain waste products that otherwise cause harm to the inner environment. For instance, reactive oxygen species (ROS) molecule is one of the natural byproducts of cellular metabolism. ROS are highly reactive molecules that contain oxygen atoms with unpaired electrons.
Although, ROS play important roles in cellular signaling and defense against pathogens, they can also cause damage to cellular components, including:
- DNA
- proteins
- lipids
Oxidative damage to DNA can interfere with its replication and transcription processes. Consequently, it leads to mutations, DNA breaks, and other genetic abnormalities.
If left unrepaired, these DNA lesions can accumulate and increase the risk of genomic instability, which can contribute to various diseases, including cancer.
Antioxidant enzymes to neutralise ROS
To counteract the situation, cells by nature posses a variety of antioxidant enzymes. Aim of these enzymes is to neutralize ROS at their source or converting them into less harmful substances. Thus, helping to protect DNA from oxidative damage.
Despite these defensive mechanisms the inherent reactivity of ROS could lead to some DNA damage. During the time, cell cycle temporarily halts certain checkpoints to allow time for DNA repair processes to take place.
During DNA repair, cells synthesize new building blocks and fill in the gaps caused by DNA damage.
In case the damage is too severe, cells undergo apoptosis to prevent the propagation of mutations. Apoptosis is process of self-destruction that occurs at cellular level.
Etoposide leads to the accumulation of ROS
An international team of researchers collaborated to investigate the metabolic enzymes and processes that play a crucial role in a cell’s DNA damage response.
The team utilized etoposide. It is a chemotherapy medication commonly used to induce DNA damage in human cell lines.
The anti-cancer drug works by causing breaks in the DNA strands and inhibits an enzyme involved in DNA repair.
Researchers observed that the induction of the drug caused generation and accumulation of reactive oxygen species (ROS) within the nucleus of the cells.
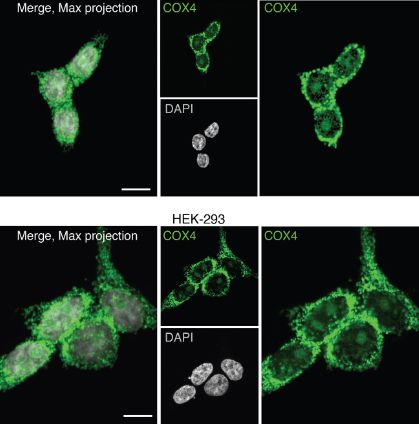
Translocation of PRDX1 from mitochondria to the nucleus
The researchers used CRISPR-Cas9 technology to identify the metabolic genes that are crucial for cell survival in response to DNA damage.
They discovered that an enzyme called PRDX1 (peroxiredoxin 1) played an important role in the cellular response to DNA damage.
PRDX1 is an antioxidant enzyme that is typically found in the mitochondria, where it helps protect cells against oxidative stress.
Interestingly, the researchers observed that upon induction of DNA damage, cells prompted PRDX1 to relocate from the mitochondria to the nucleus. This translocation allowed PRDX1 to scavenge and neutralize the reactive oxygen species (ROS) present within the nucleus, preventing further damage.
Nuclear metabolism
The findings suggest a paradigm shift in understanding of cellular biology by revealing the metabolic activity within the nucleus.
Since long, nucleus was considered as metabolically inert organelle that imports all its needs from the cytoplasm. Which, however, is not the case.
This new understanding of nuclear metabolism may have broad implications for various cellular processes, including DNA repair, gene expression, and cell signaling.
By shedding light on the metabolic activity within the nucleus, this research also adds to our knowledge of cellular complexity.
Takeaway
In cancer treatment, resistance to targeted drugs constitutes a significant obstacle.
Understanding the metabolic alterations associated with it will surely provide crucial insights for developing strategies to overcome this resistance.