Degeneration of brain cells could be a natural aging process. However, destruction or deterioration of brain cells could occur due to a wide range of internal as well as external factors. In general, brain damage refers to significant, undiscriminating trauma-induced damage.
Science has tremendously advanced when it comes to health care and disease control. There are new and radical developments in the field of neurology alone. One such progress is brain tissue reconstruction.
Brain tissue reconstruction
Brain tissue reconstruction refers to the method of restoring damaged or lost brain tissue through the regeneration or replacement of neurons and other brain cells. Stem cells, tissue engineering, and neuroprosthetics are some of the approaches that explore brain tissue reconstruction.
Although growing neuronal tissue is not an easy task because the brain has limited regenerative capacity and many of its functions are highly specialized and complex. Yet researchers at Hokkaido University have made a step in that direction.
Engineered brain tissue via hydrogel
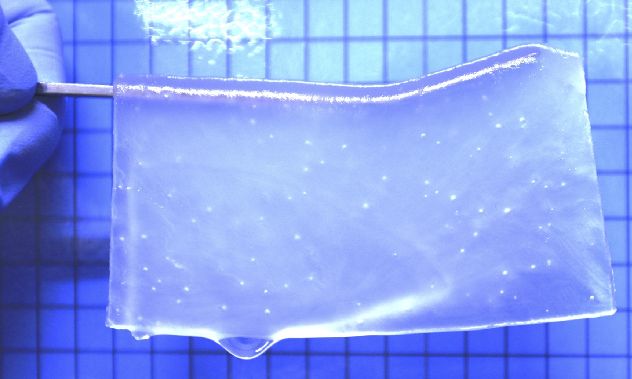
They have tried to engineer brain tissue with the help of hydrogel materials, in combination with neural stem cells.
For neural stem cell culture, they took equal parts of positive and negative charged monomers. To further optimise the properties of resultant hydrogel, researchers adjusted its the charge and stiffness, which directly impacted the cell adhesion and proliferation. The pores were set to facilitate nutrient and oxygen exchange.
Satoshi Tanikawa, the lead author explained the potential of the porous hydrogels as a scaffold for growing nerve cells.
Why hydrogels?
At the fundamental level, hydrogels are a mesh of cross-linked polymer chains, which has great water absorbance ability. And thus, they can easily mimic the extracellular matrix (ECM) of biological tissues.
The ECM is a complex network of proteins and other molecules that provides structural support and signaling cues to cells. By creating hydrogels that mimic the ECM, researchers can create a supportive environment for neuronal cells to grow and differentiate.
Host cells infiltrated the hydrogel
Once the hydrogel reached its optimal level, researchers soaked it in a growth factor serum to facilitate the growth of blood vessels. The soft solid then was embedded within the injured areas of the brain in a mouse model.
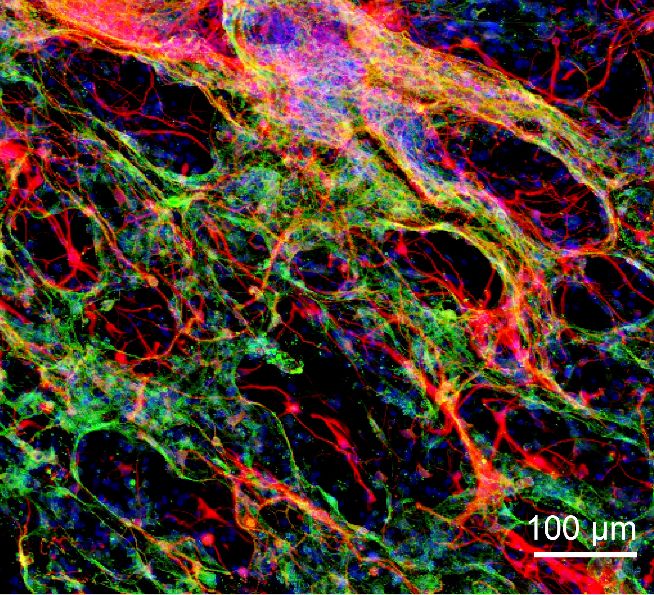
After a period of about three weeks, researchers noticed that the already present tissues – immune cells and neuronal cells – around hydrogel have paved itself into it. And also, the blood vessels had grown.
Integration between the hydrogel and host brain tissue
For the next level, neural stem cells were introduced into the hydrogel. Observations after forty days were equally astonishing. It not only showed the survival of stem cell rate high but some astrocyte cells were also formed.
Thus, there was a clear integration between the hydrogel and host brain tissue.
According to the team, the step wise approach to carry out the research has led to the expected results. The progression in a series of distinct stages include:
- first, implanting the hydrogel and
- second, transplanting the neural stem cells.
Takeaway
Overall, the development of hydrogel materials for neural stem cell culture is an active area of research. There are many ongoing efforts to optimize the design and properties of these materials for brain tissue reconstruction and other applications.
Researchers envision that their study could become the foundation for medical treatments by developing therapies that’ll involve brain tissue regeneration.
These and other developments in neurology are helping to expand our understanding of the brain and its role in health and disease, and are opening up new avenues for research and treatment.
Via: Hokkaido University