Brain cells crave immense energy to survive and communicate through connections known as synapses. These cells are like energy enthusiasts, which are busy in making way through synapses. But, in the Alzheimer’s scenario, it’s like they’re facing an energy crisis. This messes up their power production. And so, it leads to crumbling down of the synapses and consequently, our fresh memories slowly slip away.
Mitochondrial Glitch in Alzheimer’s
The Scripps Research team reported a groundbreaking study, which uncovers the energetic reactions in brain cells that set off neurodegeneration. As per the study, it’s a glitch in the energy powerhouses, the mitochondria.
In simple words, a specific mitochondrial function contributes to the development of neurodegenerative conditions. And with the help of a tiny molecule, the team was able to address the glitch in the cell’s energy powerhouses.
In models from real Alzheimer’s patients, they managed to revive a bunch of neuron connections. This hints at the potential therapy – boosting the cell powerhouses’ metabolism – for Alzheimer’s and related disorders.
‘SNO-Storm’ Unleashed in Neurons
In a recent research, Dr. Stuart Lipton and his team, (the brains behind the study at Scripps Research), uncovered a glitch. It was an abnormal tagging of nitrogen and oxygen onto a sulfur atom, creating a dysfunctional “SNO” enzyme. This S-nitrosylation reaction, a virtual “SNO-Storm,” hindered energy-making enzymes in Alzheimer’s neurons.
The team serendipitously uncovered the “SNO-tag” on energy enzymes while examining the brains of individuals with Alzheimer’s and those without any brain-related problems (gathered postmortem).
Following that, they crafted nerve cells using stem cells obtained from skin biopsies. Experimenting with a few that possessed an Alzheimer’s-related genetic mutation and others that didn’t.
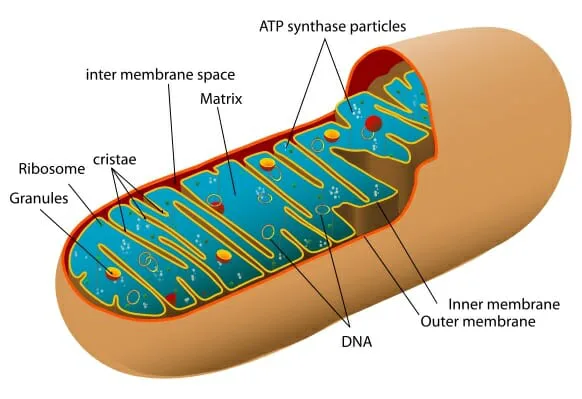
Krebs Cycle Disruption in Alzheimer’s Energy Struggle
Equipped with metabolic labels and an oxygen-measuring device, they analyzed the data. Hence, they identified distinct issues in energy production in Alzheimer’s nerve cells compared to the control group.
The researchers discovered a disruption in the neurons’ Krebs cycle. It is a cellular process in mitochondria that generates the body’s primary molecular power source, ATP.
They identified a bottleneck in the formation of a crucial molecule, succinate, which plays a vital role in ATP production. This bottleneck hindered the mitochondria’s capacity to generate the necessary energy to support neurons and their myriad connections.
Succinate Sparks Energy Revival in Alzheimer’s Neurons
The researchers hypothesized that by providing the missing succinate molecules, they could potentially revive energy production. Most importantly, it would kickstart the stalled mitochondrial Krebs cycle.
Because succinate encounters challenges in cellular movement, they chose an analog with better nerve cell membrane penetration. The approach succeeded, restoring up to three-quarters of lost synapses and preventing further decline.
While succinate isn’t currently available as a treatment, it serves as a proof-of-principle that rejuvenating the Krebs cycle is a viable approach, explained Dr. Lipton. The study’s beauty lies in demonstrating this in living nerve cells from Alzheimer’s patients. However, a much-improved compound is needed to formulate an effective drug for human use, he further added.
Takeaway
The future of this research looks promising as it may include the development of targeted therapies especially in the development of drugs aimed at improving energy metabolism in AD patients.
While the identification of protein S-nitrosylation holds promise as a potential diagnostic marker for Alzheimer’s disease. Thus, paving way for earlier and more reliable detection of Alzheimer’s disease.