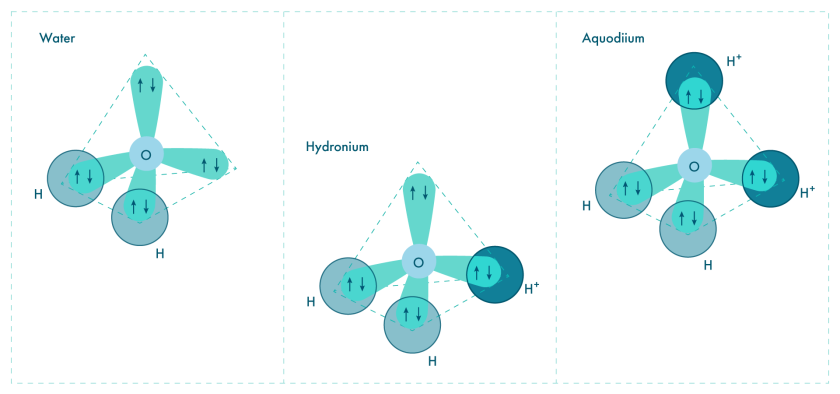
Scientists from Skoltech and their Chinese colleagues have discovered conditions that might allow for the existence of a unique ion called aquodiium. This ion is essentially a water molecule that has gained two extra protons. This means, the “regular” H₂O formula with two additional protons (H⁺), will make its chemical formula H₅O²⁺.
The researchers suggest that the aquodiium ion could be stable under the extreme pressures and temperatures found inside the ice giant planets Uranus and Neptune. This stability might contribute to their unusual magnetic fields.
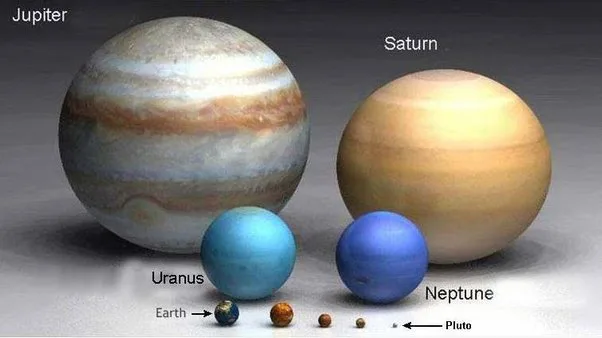
When it comes to magnetic fields, Uranus and Neptune are less well understood. While its not the case with other planets like – Jupiter, Saturn, or Earth.
Earth’s magnetic field is generated by the movement of a liquid iron-nickel alloy in its outer core. For Jupiter and Saturn, their magnetic fields are believed to originate from hydrogen that is compressed so much it behaves like a metal.
Yet, the origins and behaviors of the magnetic fields of Uranus and Neptune have remained enigmatic until now. Nevertheless, scientists have put forth one possibility: the presence of ions like aquodiium may contribute to their magnetic properties. This presents an intriguing avenue for further exploration into the mysteries of these distant ice giants.
Understanding Hydrogen Conductivity on Jupiter and Uranus
Professor Artem R. Oganov from Skoltech clarified the distinction between ionic and electronic conductivity and where the new ion fits in. As per him, in Jupiter, the layer of hydrogen surrounding its rocky core behaves similar to a liquid metal. This means that, like the molten iron flowing within Earth’s interior, the hydrogen in Jupiter can flow and conduct electricity.
The conductivity of this hydrogen results from the presence of free electrons that are shared among the densely packed hydrogen atoms. Essentially, these free electrons facilitate the flow of electric charge, much like they do in a metal.
In Uranus, the hypothesis suggests that, the carriers of electric charge are not the free electrons found in Jupiter’s hydrogen layer but rather the hydrogen ions themselves, specifically protons.
These protons may not be present solely as individual H+ ions but could also exist in various ionic forms, such as hydronium (H3O+) and ammonium (NH4+), among others.
The research has introduced a new possibility: the H4O2+ ion. This ion is particularly fascinating from a chemical perspective, as it presents unique properties that could contribute to understanding the complex behavior of Uranus’ atmosphere and magnetosphere.
sp3 Hybridization Demystified
In chemistry, sp3 hybridization involves the mixing of electron orbitals to form a specific arrangement that serves as a structural template for molecules and ions. For instance, when a carbon atom, which has four unpaired electrons, combines with four hydrogen atoms, it forms methane (CH4). In this case, the carbon atom undergoes sp3 hybridization to create a tetrahedral arrangement with the four hydrogen atoms attached to the carbon atom’s four hybrid orbitals.
Similarly, when an oxygen atom, which has two lone electron pairs and two valence electrons, bonds with two hydrogen atoms, it forms water (H2O). In this instance, the oxygen atom undergoes sp3 hybridization to create a bent molecular geometry, with the two hydrogen atoms bonded to the oxygen atom’s two hybrid orbitals and the two lone electron pairs occupying the remaining two hybrid orbitals.
Furthermore, when a hydrogen ion (H+) is added to water, it forms hydronium (H3O+). Here, the oxygen atom of the water molecule accepts the hydrogen ion, resulting in the formation of an additional bond and the creation of the hydronium ion.
Exploring Aquodiium Ions’ Role in Extreme Environments
Professor Xiao Dong from Nankai University in China explored the potential of adding an additional proton to hydronium ions.
He questioned whether it’s possible to add another proton to the hydronium ion to complete its structure. Under normal conditions, this configuration is highly energetically unfavorable. However, their calculations suggest that two factors could facilitate this process.
- Firstly, under very high pressure, matter tends to reduce its volume. In this scenario, sharing a previously unused electron pair from oxygen with a hydrogen ion (proton) becomes an efficient way to achieve this reduction. It will be similar to forming a covalent bond with hydrogen. Notably, both electrons involved in this bond come from oxygen.
- Secondly, a large number of available protons is necessary for this process. This requirement implies an acidic environment because acids, by definition, donate protons. Thus, in an acidic environment, the abundance of protons makes it more feasible to add another proton to the hydronium ion, despite the energetic challenges under normal conditions.
Using computational tools, the research team predicted that under extreme conditions of 1.5 million atmospheres of pressure and a temperature of 3000 degrees Celsius, aquodiium ions could form.
The researchers propose that aquodiium ions could play a significant role in the behavior of water-based environments under high pressure and acidic conditions, similar to those found on Uranus and Neptune.
In these extreme environments, it is theorized that aquodiium ions could form, potentially influencing various properties of the planets, including their magnetic fields. This suggests that aquodiium ions may contribute to the unique characteristics observed on Uranus and Neptune.

Takeaway
By showing how aquodiium ions could form in extreme conditions, the researchers have opened doors to observe and study the behavior of matter in the most inhospitable environments.
Plus, it’s not just about planets. This research could also give a boost to how we use computers to explore the unknowns in science. So, it’s not just about the planets; it’s about sharpening our tools for understanding all sorts of scientific mysteries.
In a nutshell, the study has the potential to make future research and technology even more exciting!



[…] How does the formation of aquodiium ions at high pressures and temperatures compare to the behavior of water under normal conditions? Ref- Aquodiium Ions Stabilized by Pressure […]